CRISPR Therapeutics AG (NASDAQ: CRSP) made worldwide headlines when it and partner Vertex Pharmaceuticals Inc. (NASDAQ: VRTX) received the first-ever FDA approval for a gene-editing therapy for sickle-cell disease (SCD) called Casgevy on Dec 8, 2023. This FDA approval was a boon to the medical sector and notably for gene therapy focused biotechs like Intellia Therapeutics Inc. (NASDAQ: NTLA) and Beam Therapeutics Inc. (NASDAQ: BEAM). bluebird bio Inc. (NASDAQ: BLUE) simultaneously received FDA approval for its gene therapy treatment for SCD called Lyfgenia on Dec 8, 2023, also.
Nvidia-like impact
The impact on the bottom line was dramatic, paralleling the impact of the artificial intelligence (AI) boom for Nvidia Co. (NASDAQ: NVDA) bottom line results. Just as AI went mainstream and viral in 2023, gene therapy and gene editing may experience its tipping point in 2024. Casgevy has now received approval in the European Union, Great Britain, Saudia Arabia and Bahrain, with more nations to come. The regulatory submission to treat SCD and TDT is under review in Switzerland and planned for Canada in the first half of 2024. Check out the sector heatmap on MarketBeat
Nvidia-like results
On Feb 21, 2024, CRISPR Therapeutics announced its Q4 2023 earnings results. The company reported EPS of $1.10, beating the 15 cents consensus analyst estimates by 95 cents or 7X. Revenues surged 3,235% to $201.2 million versus $148.72 million. Revenues are derived from milestone payments, licensing and royalties.
Casgevy is a single treatment costing $2.2 million per patient. Vertex activated 12 authorized treatment centers in the United States and signed with Synergie Medication Collective (SMC) to provide access to Casgevy. Get AI-powered insights on MarketBeat.
SMC is a contracting organization supporting payors covering nearly 100 million people in the United States. Vertex has been in correspondence with the state Medicaid organization to secure immediate coverage and reimbursement for Casgevy. The company has $2.1 billion in Pro-Forma cash and cash equivalents as of Feb 21, 2024.
Next-Gen immune-oncology and autoimmune disease candidates
CRISPR Therapeutics has ongoing clinical trials for CAR T product candidates CTX112 targeting CD19 and CTX131 targeting CD70). Pharmacology data indicate novel potency edits in CTX112 and CTX131 can lead to much higher CAR T cell expansion compared to first-gen candidates. Clinical updates for the next-gen candidates will be released later in the year.
CRISPR Therapeutics CEO Samarth Kulkarni commented, “So the picture of a Cas9, CRISPR/Cas9 goes from molecular scissors to sort of a molecular UPS truck that's bringing cargo to a particular part of the genome where you're loading an effector protein to do a desired modification on the genome and whether it's a reverse transcriptase or whether it's A MNase.”
Type 1 Diabetes (T1D) treatment clinical trial
CRISPR Therapeutics is on Phase 1 clinical trial for CTX211 for the treatment of Type 1 Diabetes (T1D), also known as insulin-dependent diabetes. CTX211 is an allogenic, gene-edited, stem-cell-derived investigational therapy for the treatment of T1D. It aims to incorporate gene-edits to make call hyperimmune and enhance cell fitness. This therapy is designed to enable patients to produce their insulin in response to glucose.
About Type 1 Diabetes
T1D usually requires lifeline insulin therapy administered through daily injections. TD1 is usually diagnosed in childhood and adolescence, but it can trigger at any age and is widely believed to be caused by genetic factors. Unlike Type 2 diabetes, T1D can't be prevented by lifestyle changes. There are approximately 1.6 million people in the United States with T1D, and nearly 10% of all diabetes cases are T1D globally.
Vertex paid CRISPR Therapeutics $170 million in upfront and milestone payments for non-exclusive rights for T1D treatment with an additional $160 million in research and development milestones along with royalties on any future products.
CTX211 comments at the Citi Virtual Oncology Leadership Summit
CRISPR Therapeutics CEO Samarth Kulkarni addressed CTX211 and the making edits to certain genes like HLAE and PD-L1 in addition to beta 2M edit. The main goal is to understand how the modified cells interact with the immune system. The goal is to get these cells to avoid being attacked by the body's immune system and, therefore, need to be cloaked or made invisible. If successful in avoiding the attack, then the modified cells have the potential to enable the production of insulin without being destroyed by the immune system.
Kulkarni summed it up, “I think the threshold question here is, can we get - can we achieve hyperimmunity or hypoimmune cells that are stealth and protect themselves against the host immune system? And if that's something that we achieve with these cells, everything else is titratable. We can dial up the cell dose for more insulin production, or we can dial it down if you need to. But the threshold question is, are these cells able to evade the immune system?”
CRISPR Therapeutics analyst ratings and price targets are at MarketBeat. CRISPR Therapeutics peers and competitor stocks can be found with the MarketBeat stock screener. CRSP shares have a 20.06% short interest.
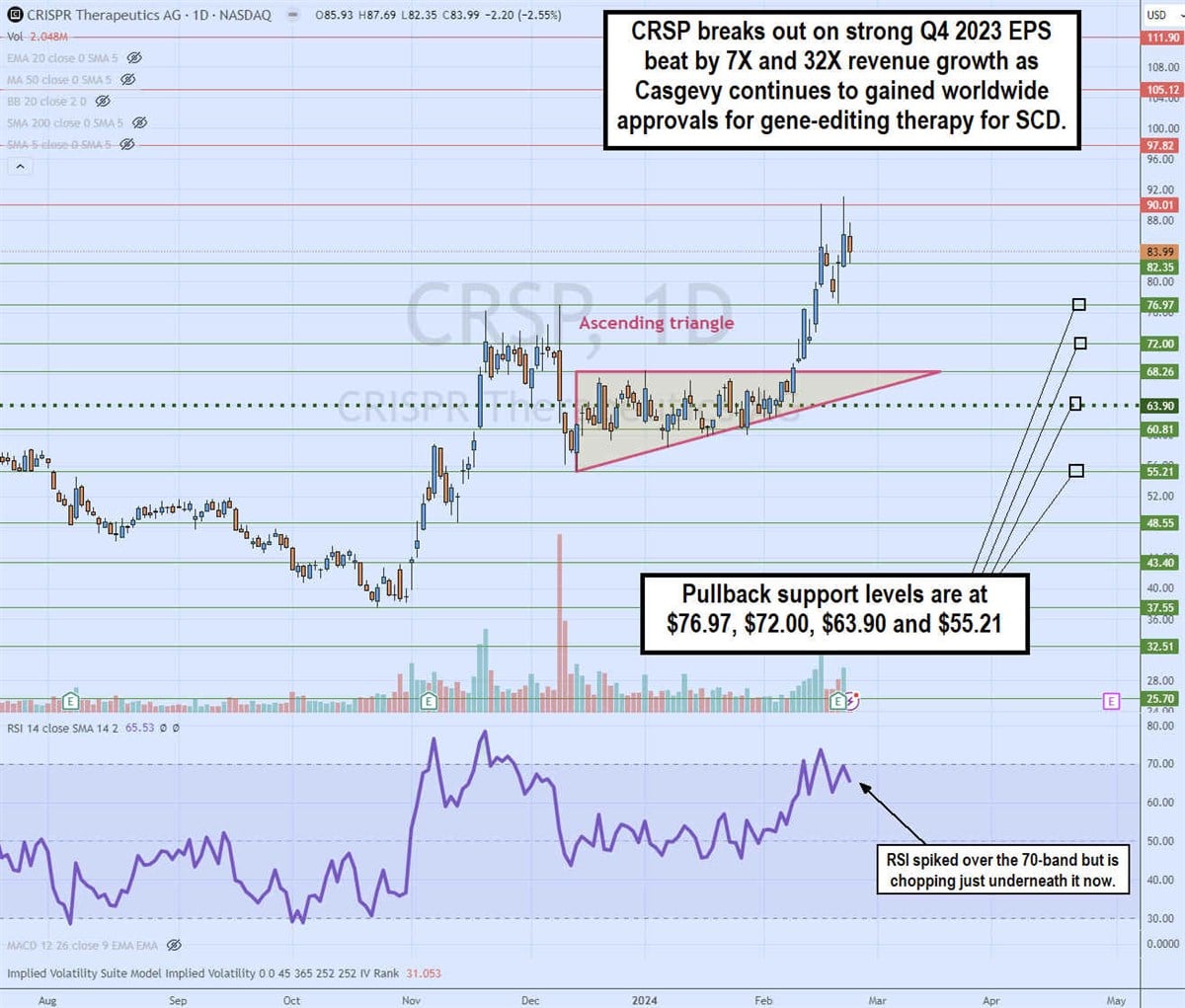
Daily ascending triangle breakout
The daily candlestick chart on CRSP illustrates an ascending triangle breakout pattern pattern. The ascending trendline formed at $55.21 on Dec 13, 2023, rising towards the flat-top horizontal trendline resistance at $68.26. The daily market structure low (MSL) was triggered at $63.90.
The breakout formed on Feb 8, 2024, surging shares up through $91.18 on its Q4 2023 earnings release. The relative strength index (RSI) spiked through the 70 bands and chopping afterward. The pullback support levels are at $76.97, $72.00, $63.90 and $55.21.





