Theralase® Technologies Inc. is currently seeking partnerships / licensing opportunities in the commercial development of this latest discovery
TORONTO, ON / ACCESSWIRE / September 3, 2024 / Theralase® Technologies Inc. ("Theralase®" or the "Company") (TSXV:TLT)(OTCQB:TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light, radiation, sound and/or drug-activated small molecules and their formulations, intended for the safe and effective destruction of various cancers, bacteria and viruses, is pleased to announce that it's lead drug formulation, Ruvidar TM , has been demonstrated preclinically to be more effective in the destruction of the Herpes Simplex Virus 1 ("HSV-1") than the currently approved standard of care drug, Acyclovir.
Acyclovir is an antiviral drug used to slow the growth and spread of the HSV-1 virus in the body. Acyclovir will not cure herpes, but it can lessen the symptoms of the infection. It is used to treat infections caused by herpes viruses, such as genital herpes, cold sores, shingles and chicken pox.
The globalantiviral drugs market sizeis expected to be worth around $ USD 71.1 Billion by 2032.
An estimated 3.7 billion people under age 50 (67%) globally have HSV-1, the main cause of oral herpes.
As an indication of the impact that a virus can have on the human population, since early 2020, the SARS-CoV-2 virus (COVID-19) has been estimated to have been responsible for over 250 million infections and 5 million deaths.
In previous experiments, conducted at the University of Manitoba, it was demonstrated that the light-activated small molecule Ruvidar TM was highly effective in inactivating numerous viruses.
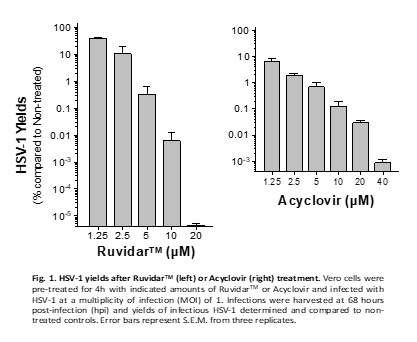
The latest research now demonstrates that Ruvidar TM is more potent than Acyclovir, the gold-standard anti-herpetic treatment, in destroying an ongoing HSV-1 infection. See Figure 1.
Another very important observation from the experiment is that Acyclovir was unable to prevent HSV-1 replication, if added one day after infection; however, Ruvidar TM was able to prevent HSV-1 replication by 10 million-fold when added 1 day after infection. See Figure 2.
In other words, from a clinical perspective, if a patient has pre-existing HSV-1, then Acyclovir would be unable to prevent replication of the virus; however, Ruvidar TM would be extremely effective.
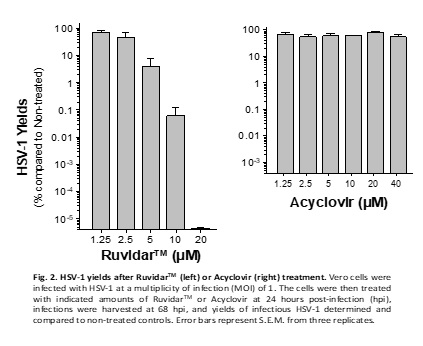
Kevin Coombs, B.A., M.A., Ph.D., professor at the Max Rady College of Medicine, Medical Microbiology and Infectious Diseases, University of Manitoba stated, " We previously demonstrated that Ruvidar TM was highly effective in inactivating numerous viruses, when used alone or activated by light. We have now demonstrated that Ruvidar TM alone is more effective than Acyclovir, the gold-standard anti-herpetic treatment, in destroying an ongoing HSV-1 infection. In a tissue culture model of HSV-1-infected cells, in which the cells were pre-treated with the drugs, before infection, it required 20 micromolar of Acyclovir to inhibit 99.95% HSV-1 replication, but conversely only 10 micromolar of Ruvidar TM to inhibit more than 99.99% HSV-1 replication. In further analysis, 20 micromolar of Ruvidar TM was able to inhibit more than 10 million-fold HSV-1 replication. Furthermore, Acyclovir was unable to prevent HSV-1 DNA replication, if added one day after infection; whereas, 10 micromolar of Ruvidar TM was able to inhibit more than 99.9% HSV-1 replication and 20 micromolar of Ruvidar TM was able to inhibit more than 10 million-fold HSV replication, even when added one day after infection. This exciting data could lead to the development of novel broad-spectrum antiviral approaches focused on prophylaxis (prevention of disease) and treatment of diseases induced by various individual viruses or virus families."
Dr. Arkady Mandel, M.D., Ph.D., D.Sc., Chief Scientific Officer of Theralase® stated, "Most viruses can cause serious and fatal illnesses and it is important to define those situations, where antiviral use is warranted and of proven benefit to minimize the toxicity and viral resistance of vaccines, which has been described for nearly all antiviral drugs. We are very pleased by the outcomes of the latest experiments conducted by Dr. Coombs, demonstrating that Ruvidar ™ alone can destroy viruses at low concentrations, preclinically. The antiviral activity of Ruvidar™ alone surpassed the antiviral activity of Acyclovir and can be even further optimized through activation, such as light, radiation, sound or a drug. It is well known that the transferrin receptor ("TfR") is an alternative target for viruses to penetrate mammalian cells. The literature strongly suggests that the TfR pathway is what a virus uses to enter and infect a cell. In previous Theralase ® research, it was demonstrated that Ruvidar™ combined with transferrin ("Tf") (Rutherrin®) was able to utilize the TfR pathway to penetrate a cell; hence, Ruvidar™ and a virus are in competition for the same TfR receptor. This theoretically allows Ruvidar™ the ability to block or significantly reduce the infectivity of the virus, as they compete for the same "door" into a cell, suggesting that Ruvidar™ could be effectively used not only as a treatment to destroy viral infections, but also as a prophylactic treatment (to prevent disease). On a final note, antiviral Ruvidar™ may find an additional clinical application in patients with cancer and those undergoing hematopoietic cell transplantation for prevention of infection from viral agents."
Roger DuMoulin-White, B.E.Sc., P.Eng., Pro.Dir., President and Chief Executive Officer of Theralase® stated, " This latest research continues to strengthen what we already know, Ruvidar™ is a very potent drug in the destruction of cancer, viruses and bacteria on its own and is further enhanced by light, radiation, sound or drug activation. Based on this latest research, Theralase® plans to commence seeking a partner / licensing opportunity in the development of Ruvidar™ for both a topical and oral treatment for the prevention and treatment of herpes simplex. "
About Theralase® Technologies Inc.:
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light, radiation, sound and/or drug-activated small molecule compounds, their associated drug formulations and the light systems that activate them, with a primary objective of efficacy and a secondary objective of safety in the destruction of various cancers, bacteria and viruses.
Additional information is available at www.theralase.com and www.sedar.com
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward-Looking Statements:
This news release contains "forward-looking statements" within the meaning of applicable Canadian securities laws. Such statements include; but, are not limited to statements regarding the Company's proposed development plans with respect to small molecules and their drug formulations. Forward-looking statements may be identified by the use of the words "may," "should", "will", "anticipates", "believes", "plans", "expects", "estimate", "potential for" and similar expressions; including, statements related to the current expectations of Company's management for future research, development and commercialization of the Company's small molecules and their drug formulations, preclinical research, clinical studies and regulatory approvals.
These statements involve significant risks, uncertainties and assumptions; including, the ability of the Company to fund and secure the regulatory approvals to successfully complete various clinical studies in a timely fashion and implement its development plans. Other risks include: the ability of the Company to successfully commercialize its small molecule and drug formulations, the risk that access to sufficient capital to fund the Company's operations may not be available on terms that are commercially favorable to the Company or at all, the risk that the Company's small molecule and drug formulations may not be effective against the diseases tested in its clinical studies, the risk that the Company's fails to comply with the terms of license agreements with third parties and as a result loses the right to use key intellectual property in its business, the Company's ability to protect its intellectual property, the timing and success of submission, acceptance and approval of regulatory filings. Many of these factors that will determine actual results are beyond the Company's ability to control or predict.
Readers should not unduly rely on these forward-looking statements, which are not a guarantee of future performance. There can be no assurance that forward-looking statements will prove to be accurate as such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause actual results or future events to differ materially from the forward-looking statements.
Although the forward-looking statements contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements.
All forward-looking statements are made as of the date hereof and are subject to change. Except as required by law, the Company assumes no obligation to update such statements.
For investor information on the Company, please feel to reach out Investor Inquiries - Theralase Technologies.
For More Information:
1.866.THE.LASE (843-5273)
416.699.LASE (5273)
www.theralase.com
Kristina Hachey, CPA
Chief Financial Officer
X 224
khachey@theralase.com
SOURCE: Theralase Technologies, Inc.
View the original press release on accesswire.com





